Newly identified oral stem cell key to wound healing
by Catherine Evans, Ph.D./National Institutes of Health
At a Glance
- Researchers found a unique population of stem cells in the roof of the mouth that quickly respond to stress from chewing and injury.
- The findings in mice could help inform efforts to improve wound healing throughout the body.
Stem cells live in many types of adult tissues, including the lining inside the mouth, called the oral epithelium. These oral stem cells can divide throughout life to replenish other cells that become damaged or diseased.
A team led by Drs. Kevin Byrd and Scott Williams of the University of North Carolina at Chapel Hill examined stem cells in the oral epithelium under normal conditions and in response to stress. The research was supported by NIH’s National Institute of Dental and Craniofacial Research (NIDCR), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and National Cancer Institute (NCI). Results were published in Cell Stem Cell on December 5, 2019.
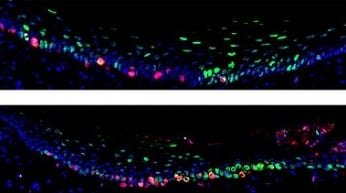
The scientists labeled and tracked oral epithelial stem cells in mice and compared cell behaviors across different tissues in the mouth. The experiments revealed a new type of stem cell in the roof of the mouth, the hard palate. These so-called “slow-cycling” cells divide infrequently and reside in specific areas on the ridges of the hard palate.
Next, the scientists examined the stem cells’ reactions to different levels of physical stress. In response to a small puncture wound in the hard palate of mice, the slow-cycling cells began rapidly dividing and migrated into the injury to renew the tissue. Feeding mice soft food (compared to a normal hard-pellet diet) evoked an opposite reaction: the stem cells divided less frequently.
By analyzing the cells’ RNA sequences, the scientists identified a gene, Lrig1, which showed high activity, or expression, in the slow-cycling stem cells. LRIG1 expression decreased near wounds in the hard palate and increased in response to a soft diet. These findings suggest that LRIG1 plays an important role in keeping the cells dividing slowly under low-stress conditions. As further evidence, mice genetically engineered to lack Lrig1 had increased stem cell division.
“Overall, the results indicate that oral epithelial stem cells in the hard palate are sensitive to both genes and the environment, responding quickly to daily challenges such as eating and to higher-stress events like injury,” Byrd says. “This knowledge could help scientists better understand how we get oral cancer, which is rare in the hard palate. It could also inform efforts to improve wound healing and tissue repair throughout the body.”

Carol graduated from Riverside White Cross School of Nursing in Columbus, Ohio and received her diploma as a registered nurse. She attended Bowling Green State University where she received a Bachelor of Arts Degree in History and Literature. She attended the University of Toledo, College of Nursing, and received a Master’s of Nursing Science Degree as an Educator.
She has traveled extensively, is a photographer, and writes on medical issues. Carol has three children RJ, Katherine, and Stephen – one daughter-in-law; Katie – two granddaughters; Isabella Marianna and Zoe Olivia – and one grandson, Alexander Paul. She also shares her life with her husband Gordon Duff, many cats, and two rescues.
ATTENTION READERS
We See The World From All Sides and Want YOU To Be Fully InformedIn fact, intentional disinformation is a disgraceful scourge in media today. So to assuage any possible errant incorrect information posted herein, we strongly encourage you to seek corroboration from other non-VT sources before forming an educated opinion.
About VT - Policies & Disclosures - Comment Policy




Epigenetics seems to offer a wide range of possible new therapies.
Comments are closed.